Biology concepts – salts in biology, osmotic potential, action potential, transpiration
We have learned that one of the crucial functions of water in living organisms is to help regulate the salt concentration in and between the cells (Gimme Some Dihydromonoxide). But why do living things require salts? We all know that we must have a source of salt (sal in Latin) in our diet or we die; the Romans gave it so much importance that part of a soldiers pay was to be used specifically for buying salt – his salary. But what are its functions?
Water tends to flow from where salts are in low concentration (high water concentration) to where salts are high concentration (low water concentration). Just like other molecules, water diffuses to where its concentration is lower (It’s All In The Numbers-Sizes in Nature). Osmosis (osmo = push in Greek) is the special name given to the diffusion of water, for every other molecule it is just called diffusion.
Too much salt is destructive to cells and organisms, so water helps control the salt held in the body. On the other hand, too much water is also bad for living things (water toxicity), so salts help to control the water concentration. Together, this ratio of salt and water inside and outside of the cell leads to a controlled imbalance called the osmotic potential of the cell. Every living thing has systems to maintain this osmotic potential within a small range (osmoregulation, we will discuss this in more detail soon).
 |
The osmotic potential is measured in units of pressure (bars). It is equal to the amount of water that will move in response to a difference in solute concentration across a membrane. |
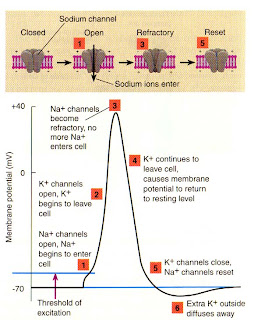
When in water, sodium chloride (NaCl, table salt) dissociates into Na+ and Cl- ions, and it is these ions, along with K+ (potassium ion from KCl) that perform many functions in living organisms. Sodium is 10x more concentrated outside the cell, while potassium is 20x more concentrated inside. The slight difference in the charges of the two ions (and the fact that most Cl- is outside cells) sets up a membrane potential in cells.
An important function of this membrane potential is in the neuron (nerve cell), as rapid reversal of the potential along the cell membrane (through ion specific channels) produces an electrical current that we know as the action potential (neural impulse). It is the rapid change in concentrations of Na+ and K+ cations (positively charged ions) inside and outside of the neurons that sends the messages from our muscles to our brains and back, as well as all the thought processes in our brain.
Salt's importance is illustrated when their concentrations get out of whack. Too little salt produces symptoms similar to dehydration, with cramping, nausea and confusion. Too much salt results in hallucinations and insanity. The classic example of too much salt intake is being lost at sea. Not having a supply of freshwater, people may start to drink seawater. The salt concentration is too high; their kidneys can’t get rid of all the excess, and the action potentials in the brain begin to misfire. People will see things that aren’t there, and will make critically bad decisions. Many end up swimming away from relative safety and subsequently drown.
We can get rid of some salt through our skin. Is your dog is happy to see you when licking your face after you arrive home, or does he just want the salt? Athletes will often eat bananas to augment their potassium stores and keep the cramps away after exercising. They should really follow that run with a bowl of lima beans; they have much more potassium.
However, munching on black licorice is alot like running a long distance. Glycyrrhizin is the main glycoside (a sugar bound to a non-carbohydrate) in licorice root and is 20x sweeter than sucrose. Glycyrrhizin prevents potassium reuptake in the kidney, so you end up urinating out most of your potassium stores. You could cramp up due to excessive snacking.
Na+ and K+ work in muscle function; cramping and paralysis may result from too little or too much salt. Your heart is a muscle, so changes in salt concentration in the cell can cause heart attacks as well. Many a mystery movie has included the injection of potassium chloride to induce a heart attack. Sodium and potassium cations help maintain proper blood pressure, proper acid/base levels, and proper movement of carbon dioxide from the blood to the lungs. There are precious few functions in which these positive ions don’t play a role.
 |
Collagen and elastin help to make your skin and joints pliable. O.K., maybe not this elastic – this is the result of Ehlers-Danlos syndrome, which is often a genetic disease. |
When we think of salt, we usually think of table salt (NaCl), but there are more functions for K+ than there are for Na+, and it is present in higher concentrations in the cell. Potassium is important for the formation and crosslinking of collagen and elastin proteins. These connective tissue proteins hold all your tissues together; they keep your skin from tearing when someone pokes you in the arm, and allow your lungs to expand without ripping when you inhale. So K+ is pretty important even when not working with Na+. It is interesting then that potassium is the only major mineral nutrient for which there is not a recommended daily allowance.
Remember that we often take in these salts as NaCl or KCl. Does the Cl- play a role in organism function? – you bet it does. Chloride anion (a negatively charged ion) is used to produce the hydrochloric acid (HCl) that breaks down the food in our stomachs. Chloride also works in the immune system, hypochlorite (the same active molecule as in bleach) in the white blood cells helps to kill infectious agents and activates other immune system molecules. Chloride is required for the uptake of vitamin B12 and iron and helps control your blood pressure; therefore, Cl- isn’t just that other ion that comes in with Na+ or K+ (or Ca2+).
Chloride ion is elemental chlorine that has gained one electron. This doesn’t seem like much of a change, but it is the difference between life and death. Chlorine itself is a yellowish green gas and it can kill you in a matter of seconds. Chlorine really wants that extra electron, and it doesn’t care if it has to rip it from your lung proteins to get it. When you breathe in chlorine, it reacts with the water in your lungs to produce hydrochloric acid that eats away the cells. It will also react with almost any carbon-containing molecule and further destroy the lung tissue. It was suggested during the American Civil War that chlorine gas could be useful, but it wasn’t until World War I that it was used as a weapon.
Chlorine is poisonous, but we use it to disinfect drinking water and pools. When diluted greatly in water, chlorine does not have the strongly deleterious effect on our cells as it does as a gas, but can still react with and kill microorganisms. Chlorination of water began in the Chicago stockyards around 1908, when the decaying meat and gut bacteria were getting into the drinking water and making the residents sick. The bleach used to disinfect surfaces is much the same as the chlorine used to disinfect 75% of the drinking water in the U.S.; it’s just there in lower concentration. Now chlorine is used in pools as well, and you know it is working because your eyes get red and sting.
There are no exceptions to the rules of salt requirements (weird, isn’t it). All living things need to take in Na+, K+, Ca2+, and even Cl-. Plants use potassium and sodium for water balance, especially to bring morphologic changes like the blooming of flowers. These cations, along with chloride, work in the opening and closing of pores in the leaves (stomata) for the uptake of carbon dioxide and the release of oxygen and water during transpiration (Gimme Some Dihydromonoxide), and in the chemical splitting of water during photosynthesis. It seems that other organisms rely on these ions even more than animals.
All bacteria require potassium and sodium for osmotic regulation and cellular activities.
As the concentration of Na+ in a bacteria’s environment goes up, its dependence on Cl- becomes greater. Fungi, protists, and even viruses depend on salts to remain alive, even though viruses are technically not a form of life. Viruses carry nucleic acid, and salts are needed to balance the charges of the DNA or RNA so it can be stuffed into the viral package, a function within the area of molecular biology. Molecular biology involves replication of DNA, the transcription of DNA to RNA, and the activities of RNA translation to proteins. K+, Cl-, and Na+ are involved in all these areas. In a feedback mechanism, salt ions control the switches that turn on genes that then control the levels of the ions. If one ion is too high, it will turn on the genes that code for proteins which remove that ion from the cell. Isn’t evolution nifty?
Tightly regulating salt concentration in the cell is important for life, and we have to drink water (kangaroo rats excepted) in order to stay alive. These are the peanut butter and jelly of biology and we will start to see how they work together next time.
For more information and classroom activities on salts in biology, osmotic potential, action potentials, or chloride ion in biology, see:
Salts in biology –
Osmotic potential –
Action potential –
Chloride in biology -
stomata –
http://www.apsnet.org/edcenter/intropp/topics/Pages/OverviewOfPlantDiseases.aspx